Citizens Law Firm on What to Do About Defective Medical Devices
Like all manufacturers, medical device companies have a responsibility to ensure that their products have been designed and manufactured correctly, and that the products are free from defects. More importantly, when a defect is discovered, medical device manufacturers have a duty to warn patients, the medical community, and the US Food & Drug Administration (FDA).
The latter is extremely important when it comes to medical devices, as many are physically implanted in patients and any “defect” has the potential to require surgery, or even cause serious injury or death.
Manufacturers May Be Liable for Your Injuries
There are many ways medical device manufacturers can be held liable for injuries you suffer after using their product, including failing to properly:
- Design the device.
- Test the device.
- Manufacture the device.
- Warn about issues with the device.
- Recall the device when warranted.
Sadly, many medical device manufacturers rush their products to market and fail to warn of known defects that will affect their bottom line. The result? Litigation.
Defective Medical Device Litigation
Medical device manufacturers often put profits before patient safety. This has led to serious injuries, deaths, and a great deal of defective medical device litigation, including devices such as:
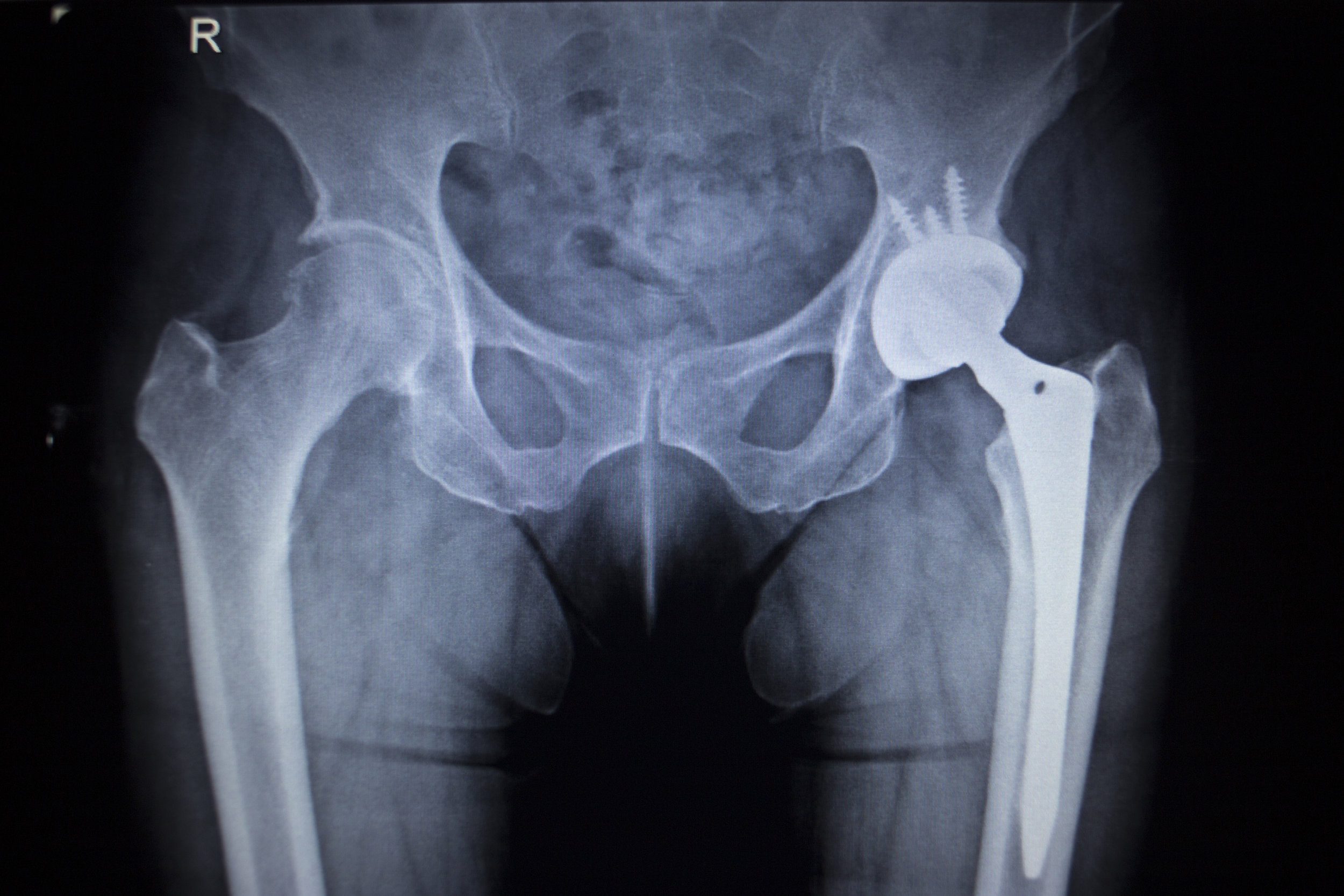
- Hip Implants: DePuy Orthopaedics, a subsidiary of Johnson & Johnson (J&J), introduced a series of metal-on-metal hip implants that resulted in metal poisoning and revision surgery due to the loosening of the implant or joint dislocation. Evidence showed that manufacturers knew about the defects but continued to sell and market the implants. They settled thousands of lawsuits in 2013 for $2.5 billion.
- Transvaginal Mesh: Ethicon, another subsidiary of J&J, introduced vaginal mesh implants designed to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI), conditions experienced by older women following menopause, childbirth, or a hysterectomy. Thousands of women filed lawsuits alleging organ perforation, vaginal bleeding and scarring, mesh erosion, and severe pain. Manufactures have paid millions of dollars in verdicts and settlements already and thousands more lawsuits are pending.
- Power Morcellators: Ethicon is one of a number of companies that manufactured power morcellators—devices used during hysterectomies and fibroid surgeries. However, the devices can cause hidden uterine cancer to spread. The FDA issued a warning that morcellators could increase the risk of cancer and the Federal Bureau of Investigation (FBI) is currently investigating the manufacturer about known risks.
Sadly, these are only three examples of medical devices whose defects have led to significant litigation. Others include IVC filters, pacemakers, pain pumps, heart valves, knee replacements, defibrillators, and more.
Medical Device Approval Process Dangerous to Consumers
Even though technology has allowed medical device manufacturers to design more innovative products, defective devices continue to reach consumers. Why? Medical device companies often take advantage of the FDA’s limited ability to police the industry. Manufacturers generally receive FDA product approval in two ways:
- PMA. Pre-market approval (PMA) is the most stringent type of device marketing application required by FDA. The process, which takes 180 days at a minimum, requires manufacturers to provide a significant amount of detail about the device—including information on clinical trials. It is a complex process that allows the FDA to do its own research before the device is placed into the consumer market.
- 510(k). A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device. Unlike a PMA, the 510(k) process is much simpler. So, as long as the device is no more dangerous than something already on the market, the FDA is likely to approve it.
Sadly, many manufacturers take advantage of the latter option. While it’s less expensive for them, it can be very dangerous for consumers.
Have You Been Injured?
If you’ve been injured by a defective medical device, you may be entitled to compensation, including payment for your medical bills, lost wages, pain and suffering, medical monitoring, and more. Keep in mind that every situation is different and every potential lawsuit is subject to a specific Statute of Limitations—some as short as one year.
Citizens Law Firm: Let Us Help You Understand Your Rights!
Don’t miss out on your ability to receive compensation for your defective medical device injuries. At the Citizens Law Firm, we will help you understand your rights, fight for your rights, and obtain the compensation you and your family deserve. Call us today at (844) LAW‐CALL or complete our simple Free Case Evaluation form and we’ll call you.